Aerobic performance reflects the ability of animals to carry out important tasks requiring high rates of aerobic metabolism, such as sustained locomotion or thermogenesis (body heat generation). Aerobic performance requires high rates of O2 supply to metabolically active tissues, supported by a physiological system termed the O2 transport pathway. The overall capacity of the O2 transport pathway sets the ceiling for sustained aerobic performance, which is called aerobic capacity and is often measured as the maximal rate of O2 consumption (VO2max).
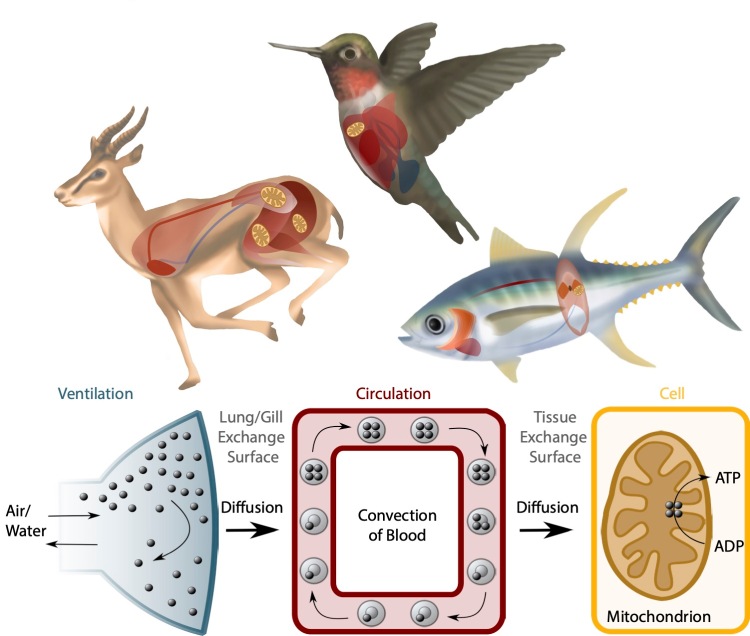
The O2 transport pathway moves O2 from the environment to tissue mitochondria through a series of physiological steps – namely, ventilation, diffusion into the blood, circulation, diffusion into peripheral tissues and mitochondrial O2 utilization (Scott and Dalziel 2021). Illustration by Samantha Lau.
Aerobic performance contributes to fitness in many animals, and the function of the O2 transport pathway can be a key determinant of how animals cope in challenging environments. Studies of the O2 transport pathway can also answer fundamental questions about how organisms evolve (see Comparative & Evolutionary Physiology). Our research uses a comparative approach in different vertebrate groups to examine these issues.
High-Altitude Adaptation in Deer Mice
Most of our research on aerobic performance and the O2 transport pathway strives to explain the integrative physiological mechanisms of evolutionary adaptation and phenotypic plasticity (e.g., acclimatization, developmental plasticity) in deer mice (Peromyscus maniculatus) at high altitude. High-altitude environments are much colder than those at sea level, increasing the O2 demands of thermogenesis, but high altitude also has less O2 available to support these high O2 demands (hypoxia). We use a comparative approach to uncover how adaptation and plasticity affect aerobic performance and the O2 transport pathway, using lab colonies of deer mice derived from wild populations at high altitude (4300m elevation in the Rocky Mountains) and low altitude (see Comparative & Evolutionary Physiology). We have shown that high-altitude populations have evolved increased aerobic capacity in hypoxia to support thermogenesis and exercise, and that aerobic capacity is also increased by plastic responded to hypoxia and/or cold exposure during adulthood and early life. Our ongoing work is uncovering the changes across the O2 transport pathway that underlie these differences.

Recent work has shown that changes across the O2 transport pathway contribute to evolved and plastic increases in aerobic capacity in high-altitude deer mice, and that the relative influence of changes at one step in the O2 pathway (e.g., circulation of O2 bound to haemoglobin in the blood) depends upon the state of other steps in the O2 pathway (e.g., diffusing capacity of peripheral tissues) (Tate et al. 2020; Wearing et al. 2021).
Chronic hypoxia at high altitude can constrain tissue O2 supply, but it can also initiate maladaptive physiological adjustments that contribute to pathology and disease (e.g., mountain sickness in humans, brisket disease in cattle). Such maladaptive responses can result when a physiological response that evolves to carry out a particular function in one environment leads to an off-target physiological response that reduces health and fitness in a novel environment. It is expected that maladaptive responses should be strongly selected against when animals adapt to novel environments. Indeed, our recent work has shown that maladaptive responses to chronic hypoxia are strongly attenuated in high-altitude deer mice.
Hypoxia Tolerance in Fish
Hypoxia can be a significant challenge in many aquatic environments, and can arise from both natural and anthropogenic causes. Some aquatic environments can experience prolonged periods of hypoxia (e.g., during ice cover over winter months), while some others may undergo dynamic cycles of intermittent hypoxia (e.g., diel cycles of biological activity). Hypoxia can be severe in some aquatic environments, and yet some species of fish are able to cope and overcome the significant challenge to balancing tissue O2 supply and demand. We have shown that the evolution of hypoxia tolerance in fish is associated with changes across the O2 transport pathway. We have also shown that chronic exposure to hypoxia leads to plastic adjustments that improve hypoxia tolerance, but the mechanisms used to cope with hypoxia depend strongly on the pattern of hypoxia exposure (i.e., sustained hypoxia versus diel cycles of intermittent hypoxia). Hypoxia tolerance also depends on thermal history, and we have shown that the effects of temperature on hypoxia tolerance depend on the pattern of temperature variation (i.e., constant temperature versus diel temperature fluctuations).